Denitrification of nitrate down to molecular nitrogen involves a stepwise reaction with the intermediates nitrite - NO - N2O. Each step requires a unique reductase. Nitrate reductase in facultatively anaerobic bacteria is a transmembrane protein associated with a nitrate/nitrite antiporter. The first reaction intermediate nitrite is expelled to the periplasm, where the enzymes for the next two steps are located (both nitrite and NO are poisenous to the cytoplasm).
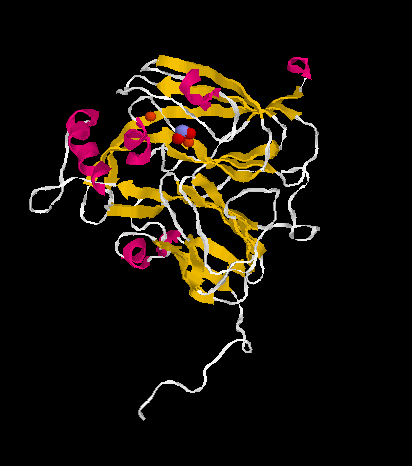
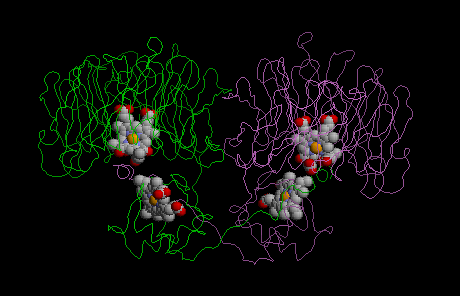
To date two classes of dissimilatory nitrite reductases are known: in class I enzymes copper is used as cofactor, in class II heme is at work. Still another kind of nitrite reductases containing hexaheme are found in the ammonification part of the nitrogen cycle.
The molecular structures of both class I and lass II enzymes could be elucidated.
Class II - enzymes catalyze both the one electron reduction of nitrite to NO
NO2- + 2 H+ + e- -> NO + H2O
and the four electron reduction of oxygen to water
O2 + 4 H+ + 4 e- -> 2 H2O
Because of the heme contained in the enzyme it has been previously named cytochrome cd1. However, the reduction of nitrite is about ten times more efficient in denitrifying bacteria and therefore to be regarded as the physiologically relevant reaction. Class I enzymes containing copper are inactivated by oxygen in a reaction yealding hydrogen peroxide.
| NIR class I | NIR class II | |
 |
 |
|
| structural details | structural details |
The NO released during nitrite reduction is further reduced to dinitrogen oxide by nitrogen monoxide reductases
2 NO + NADH + 2 H+ -> N2O + NAD+ + H2O
This reaction involves no other protein as the electrons are exchanged directly between NAD and the prosthetic heme. The enzyme from the denitrifying fungus Fusarium oxysporum could be crystallized both in the free form and in a form occupied with substrate.
| NOR F. oxysporum |
 |
| structural details |