Proteasomes (EC: 3.4.99.46) are the main enzymes of the non-lysosomal pathway of protein degradation in cells of higher organisms (ATP-dependent ubiquitine conjugate pathway). Their purpose is to degrade misfolded and short-lived proteins. The latter means participation in the regulation of the cell cycle both in plants and animals. In plants proteasomes are involved in the defense of infections (tobacco/TMV). In animals they produce peptides, which are presented by the major histocompatibility complex I to the immune system in order to induce antibodys. Generally the peptides produced are six to nine amino acids in length.

The active parts of proteasomes sediment in an ultracentrifuge with a coefficient of 20S. They are constituted by four stacked rings of protein comprised of seven subunits each. The inner rings (white) are made up of type beta subunits which are catalytically active. These proteins are systhesized as precursors. Only at the time of assembly the propeptides are cut off and the active amino acid (threonine) becomes sterically accessible.
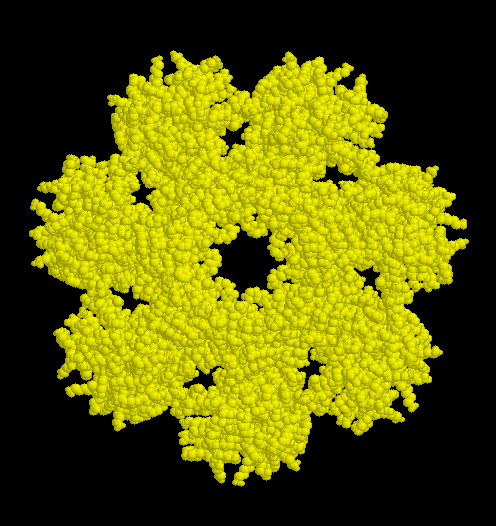
The outer rings contain proteins of type alpha. These have regulatory functions. The subunits assemble spontaneous to the circular sevenmers, to which the beta subunits are subsequentially added.
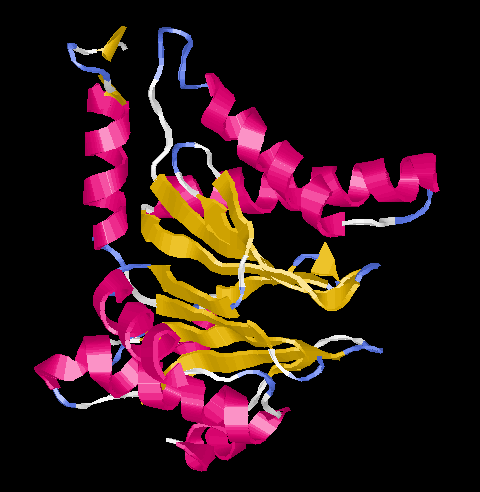
All subunits show the same folding pattern (Ntn = N-terminal nucleophile) which was found also in several other less complex enzymes. In the center there are two five-stranded beta-sheets which are surrouded by alpha-helices.
In eukaryotes the barrel of four alpha- and beta-rings is capped by protein complexes sedimenting with 19S. These complexes contain ATPases which prepare the proteins to be digested by unfolding them. These 'large' proteasomes are referred to as 26S. They were found in the cytosol and nuclei of all cells investigated.
The archaebacterium Thermoplasma acidophilum harbours 20S-proteasomes made from 14 identical alpha- and beta-subunits. These proteasomes are 11 nm in diameter by 15 nm height. Their structure was investigated by x-ray diffraction, details may be seen by cklicking here.