The term 'toxin' was originally used for filtratable ( = subcellular) substances inducing illness. The first poison recognized to be produced by bacteria was diphtheria toxin (E. Roux & A. Yersin, Institute Pasteur 1888). Today hundreds of toxins are known, which all exert some deadly effect on living cells. Each one damages cellular membranes or components thereof, or they circumvent their protective functions to damage cellular components. Toxins are proteins of quite complex structures with very specific targets. They may be classified into some groups: porin-like toxins puncture cell walls so the afflicted cell looses nutrients and experiences a severe ionic imbalance; toxins with an intracellular target form a channel in the cell wall to introduce a (proteinacous) component that switches off some cellular function; another group binds to or modifies existing ion channels and inhibits e.g. nervous exciting. Bacteriocins (in E. coli colicins) functionally belong to the group of toxins, they are very specific bactericidal agents.
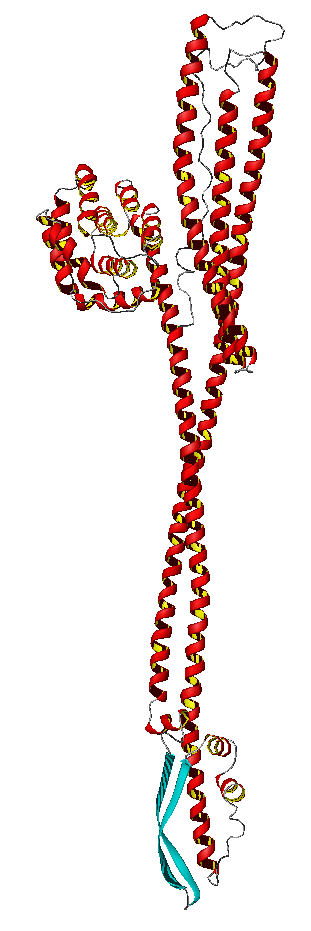 Channelforming toxins are built according to two different patterns: one type uses long alpha-helices to span membranes, the other uses extended beta-sheets wrapped into barrels functioning as pores. In both cases the producer of the toxin has to use some suicide protection: the toxin has to be excreted from the cell in an inactive form and must be allowed to become active (wall destructing) only upon reaching the destination cell. This is a multistep process. The toxin proteins are synthesized in a water soluble form and exported as such (depicted at right: Colicin Ia in the water soluble conformation). In this form they diffuse through the surrounding medium/blood circulatory system until they meet with a suitable membrane or are able to bind to a specific receptor. In some cases there follows an oligomerization. Refolding of the protein domains then turns the water soluble into a membrane insertable conformation. The refolded protein complex then is fastened to the membrane and forms a channel.
Channelforming toxins are built according to two different patterns: one type uses long alpha-helices to span membranes, the other uses extended beta-sheets wrapped into barrels functioning as pores. In both cases the producer of the toxin has to use some suicide protection: the toxin has to be excreted from the cell in an inactive form and must be allowed to become active (wall destructing) only upon reaching the destination cell. This is a multistep process. The toxin proteins are synthesized in a water soluble form and exported as such (depicted at right: Colicin Ia in the water soluble conformation). In this form they diffuse through the surrounding medium/blood circulatory system until they meet with a suitable membrane or are able to bind to a specific receptor. In some cases there follows an oligomerization. Refolding of the protein domains then turns the water soluble into a membrane insertable conformation. The refolded protein complex then is fastened to the membrane and forms a channel.
The structures of some toxins could be elucidated for the water soluble or the membrane bound conformation. Here you may see as examples
A-B toxins are composed of two components: a binding domain B mediates the transport of an enzymatically active A-domain into the target cell. The active domain is proteolytically cleaved off from the binding domain within the target cell or the toxin is composed of two separate proteins in the beginning. The group of A-B toxins harbours well known agents: diphtheria toxin modifies a rare amino acid (diphthamid) within elongation factor 2 and thereby blocks protein synthesis; cholera and pertussis toxin modify a regulatory protein of the adenylate cyclase by adenylation; shigella toxin and ricin (from plants) cleave an essential base off the 28S rRNA.
Examples for this group are
Many naturally occuring poisons contain toxins composed of peptides 60-70 aminoacids in length. The group of alpha neurotoxins blocks the action of the neurotransmitter acetyl choline by occupying the receptor (e.g. erabutoxin). Dendrotoxins block potassium channels, the scorpion toxin variant 3 interferes with sodium channels.
| Erabutoxin b from Laticanda semifasciata (sea snake) |
Dendrotoxin from Dendropsis angusticeps (green mamba) | Scorpion toxin var. 3 from Centuroides sculpturatus |
Literature:
S Olsnes et al, Protein toxins with intracellular targets, Microb. Pathogen. 8 (1990) 163-168
RJ Read & PE Stein, Toxins, Curr. Opin. Struct. Biol. 3 (1993) 853-860
CL Sears & JB Kaper, Enteric bacterial toxins, Microbiol. Rev. 60 (1996) 167-215
V Cabiaux et al, Interaction with a lipid membrane: a key step in bacterial toxins virulence, Int. J. Biol. Macromol. 21 (1997) 285-289
E Gouaux, Channel-forming toxins: tales of transformation, Curr. Opin. Struct. Biol. 7 (1997) 566-573