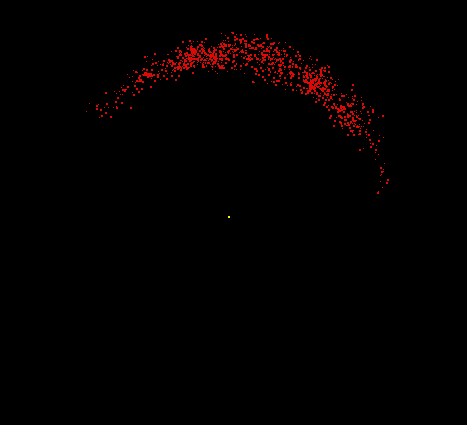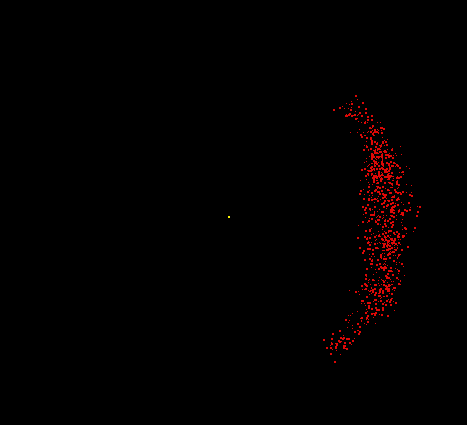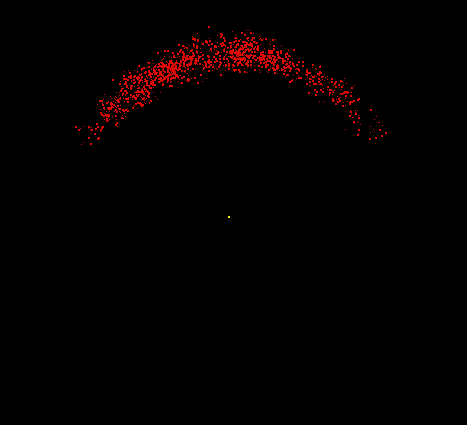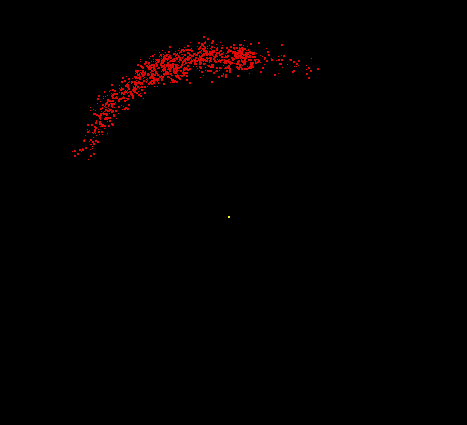
Hydrogen consists of two fundamental particles, one electron and one proton. The electron is smaller and lighter than the proton, with a mass of only 9.1 x 10-28 gram (about 1/2000 the mass of a proton). The electron, shown here as a moving cloud of red dots, is localized within a spherical probability shell surrounding the proton, the yellow dot at the center. In reality, the proton would be too small to be seen at this resolution. Most of the atom is empty space. If the hydrogen atom were magnified to the size of Yale Bowl (a stadium seating about 70,000 people), the nucleus could be represented by a basketball on the 50-yard line, and the electron could be represented by a pea whizzing around the outer rows of bleachers. Although still vastly oversimplified, this model is a reasonable analogy for the relative sizes involved.
Image by Thomas TERRY, The Biology Place.
|
|