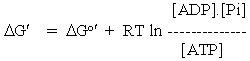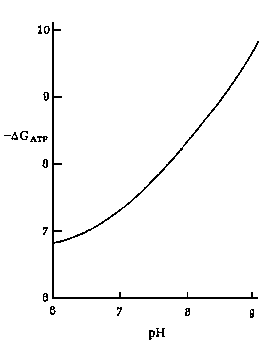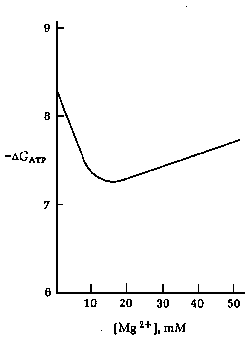Factors contributing to the free energy of hydrolysis of ATP
The overall equation for ATP hydrolysis is usually written:
ATP + H2O <=> ADP + inorganic phosphate (Pi)
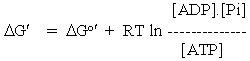 DGo' = -7.3 kcal. mol-1
Note, the H2O is ommitted from the logarithmic
term because it is the solvent with a standard
state of 1M.
(Click here for a reactant structures
in color)
DGo' = -7.3 kcal. mol-1
Note, the H2O is ommitted from the logarithmic
term because it is the solvent with a standard
state of 1M.
(Click here for a reactant structures
in color)
However, there are a number of factors which make the true situation
more complicated. Most important are:
-
The inorganic phosphate and the phosphate groups on ATP and ADP are weakly
acidic, and have pK values in the physiological range. The activities of
the different forms will vary depending on the pH of the reaction medium,
and this will alter the value for DGo.
-
HATP3- <==> ATP4- + H +; pK1'
= 6.95
-
HADP2- <==> ADP3- + H +; pK2'
= 6.88
-
H2PO4- <==> HPO42-
+ H +; pK3' = 7.20
(The effect of these pK values is that a H+ is released on ATP
hydrolysis, with a stoichiometry which approaches 1 above pK3'.
This proton release can be used to assay the reactions of ATP hydrolysis
or synthesis, or follow the kinetics if a recoding pH meter is available.)
-
The presence of Mg2+ will also modify the value for DGo,
because the different reactants form complexes with bivalent cations. For
Mg2+, the following equilibria are important:
-
Mg2+ + ATP4- <==> MgATP2-
-
Mg2+ + ADP3- <==> MgADP-
-
Mg2+ + HPO42- <==> MgHPO4
Much work has gone into the measurement of the variation of DGo'
for ATP hydrolysis with pH and Mg2+ concentration, which is
summarized in tables and graphs available in the literature. Two useful
graphs are shown below, which cover the most important physiological range.
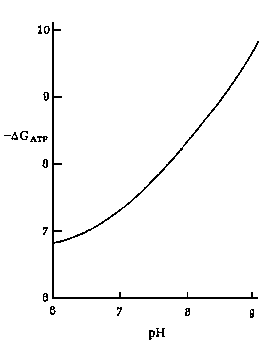
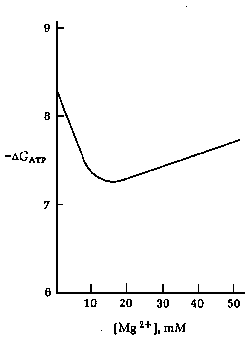
Left: Effect of pH on the free energy of hydrolysis of ATP (DGATP)
at 10 mM Mg2+.
Right: Effect of Mg2+ concentration on the free energy of
hydrolysis of ATP (DGATP) at pH 7.0.
Note that for ATP synthesis, the reaction is written in the reverse
direction, and the sign of the free energy change is reversed, so that:
ADP + inorganic phosphate (Pi) <=> ATP + H2O
DGo' = +7.3 kcal. mol-1
The modifying factors which affect the hydrolysis also effect the reverse
reaction, so the graphs above can be used to find a value for the free
energy of hydrolysis under any set of conditions, and the sign reversed
to find the free energy for synthesis of ATP under the same conditions.
©Copyright 1996, Antony
Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu