Lecture 8Electrochemical and protochemical circuits |
A cell provides power by driving a flow of current through an external circuit using the free energy available from an oxidation reduction reaction. In principle, the cell operates by connecting two half-cells of an oxidation reduction reaction like the electrochemical cell we discussed in the context of redox potentiometry. When you use a cell to provide power, the "external circuit" replaces the electrometer, and the flux is non-zero. The current is proportional to the rate of chemical reaction in the two half-cells.
The diagram below shows a circuit in which two electrochemical cells can be connected, so that the chemistry in one drives the chemistry in the other in reverse. This is what happens when a kindly friend recharges the battery in your car by connecting jumper cables from the battery in her car.

In the electrical circuit, we will assume that cell 1 is initially fully charged, and cell 2 initially fully discharged. If switch 1 is closed, cell 1 will initially generate current rapidly while charging up the capacitor; then it will discharge at a steady-state determined by the resistor value. When switch 2 is closed, cell 1 current will flow rapidly through the circuit as cell 1 provides power to recharge cell 2.
Note that an "invisible" component is implicit in the diagram,- the insulation which prevents electrons from shunting between components, except through the conductors connecting the different elements of the circuit.
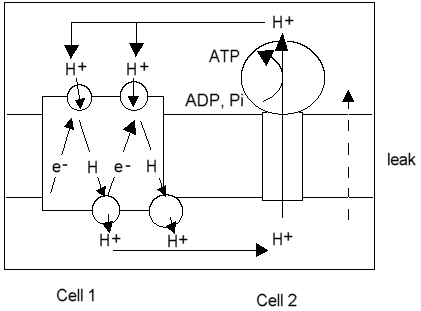
In the proton circuit, the same elements exists: The electron tranfer chain is one protochemical cell, and the ATP synthase the other. The latter is discharged when ADP is high, and ATP is low. The first cell is charged when the driving force for the electron transfer is applied,- substrate and oxygen in respiration, or light in photosysnthesis. The conductors are provided by the aqueous phases (water is a good conductor of protons), and the insulation is explicitly provided by the "coupling" membrane.