Mechanism of the Qo-site of the bc1-complex,
shown in a 3-D movie
.
The model shows two aspects of the mechanism (1) which have emerged
from consideration of the structures from Ed Berry's laboratory.
-
The mobile head of the iron sulfur protein (ISP) (red spacefilling model)
of the complex moves between docking interfaces on cytochrome b (light
blue) and cytochrome c1 (green), while the anchor (dark blue)
remains stationery. Electron transfer occurs from QH2 at the
cytochrome b interface, and to heme c1 at the cytochrome c1
interface, and the ISP moves back and forth between these sites as the
enzyme turns over. The docking position on cytochrome b is seen in stigmatellin
containing crystals, that near cytochrome c1 in native crystals.
-
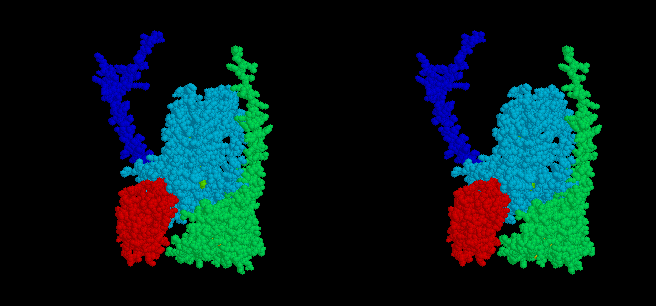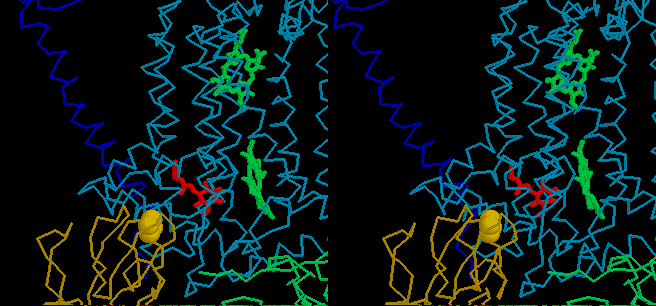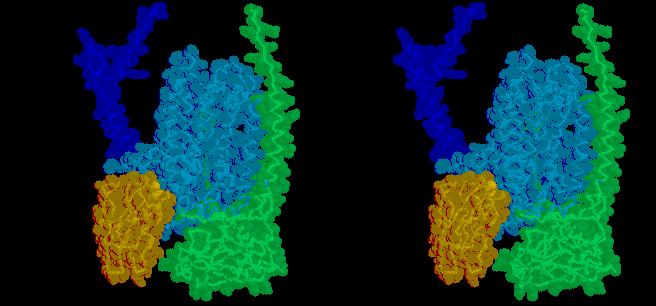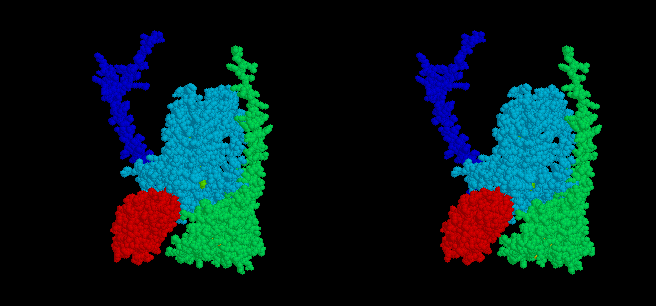
The quinone binding pocket at the Qo-site has two different
domains. At one of these (the domain distal from the heme of cyt bL),
the occupant is close to the Fe2S2 center. This domain
is occupied by stigmatellin in one of Berry's structures, and we suggest
it is the site at which the reaction complex between the enzyme, QH2
and the ISP is formed, from which QH2 undergoes oxidation. The
movie shows binding of QH2 (yellow) in the pocket, followed
by oxidation of QH2 to form the semiquinone (red), in this domain.
The second (proximal) domain, nearer to the heme of cyt bL,
is occupied by myxothiazol in another of Berry's structures. We suggest
that the semiquinone (red) moves to this site on formation, and from this
position passes its electron to the heme of cyt bL, forming
Q (blue), which then exits the site. Note that because the activation barrier
is in the reactions leading to dissociation of the reaction complex into
products (2), the movement of the ISPred away from its docking
domain on cyt b, and the movement of the semiquinone from distal to proximal
lobes, would have to occur simultaneously. For technical reasons, these
processes are shown as separate in the movie. The equilibrium constant
for semiquinone formation is extremely small (2, 3), so the semiquinone
is not detectable, even under conditions in which mass-action would favor
its production.
-
Electrons are shown as small light blue discs, and protons as smaller white
discs, leaving the site for the aqueous interface. These are shown for
illustrative purposes only. See main text for our suggestions about release
of the first proton.
Reference.
-
Crofts, A.R., Barquera, B., Gennis, R.B., Kuras, R., Guergova-Kuras, M.
and Berry, E.A. (1997) Mechanistic aspects of the Qo-site of
the bc1-complex as revealed by mutagenesis studies, and the
crystallographic structure. Proceedings of the IXth. International Symposium
on Phototrophic Prokaryotes, Vienna, Sept. 1997, (Peschek et al., eds.).
-
Crofts, A.R. and Wang, Z. (1989) How rapid are the internal reactions
of the UQH2:cyt c2 oxidoreductase? Photosynth. Res. 22, 69-87.
-
Jünemann, S., Heathcote, P. and Rich, P. (1998) J. Biol. Chem. 273,
21603-21607
©Copyright 1996, Antony
Crofts, University of Illinois at Urbana-Champaign,
a-crofts@uiuc.edu