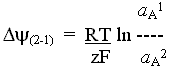Lecture 11. The proton circuit
Coupling through the proton gradient
|
Mitchell's proton pumping loops
Mitchell suggested that electron transport chains could act as proton pumps
if they contained alternate electron carrying and H-carrying spans of redox
couples arranged so as to transport electrons and hydrogen vectorially
across the membrane.
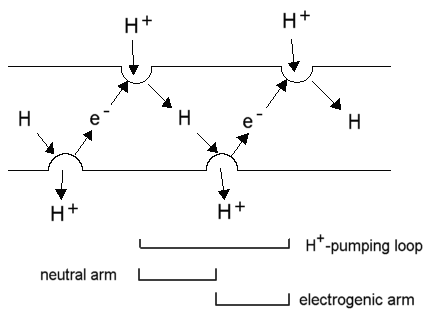
The reduction of a H-carrier by an electron carrier in one phase would
lead to uptake of H+; the diffusion of the H-carrier (as a neutral
species) across the membrane, and its oxidation on the other side by an
electron carrier would lead to release of a H+; electron transfer
back across the membrane would carry a -ve charge across the membrane in
the opposite direction, thus allowing the loop to carry 1 H+
(net) in an electrogenic proton pumping loop. The picture below shows the
Mitchellian proton pumping loops in the best defined electron transfer
chain, that for the photosynthetic apparatus of Rb. sphaeroides.
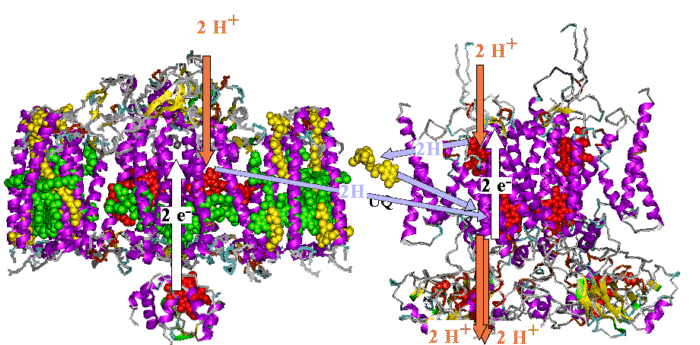
The photosynthetic chain of Rhodobacter sphaeroides, shown
as two Mitchellian proton pumping loops. The Rb. sphaeroides chain
has all components represented by models of the active components based
on X-ray crystallographic structures at atomic resolution, - the first
supramolecular machine defined at the atomic scale.
Left: The photochemical reaction center, surrounded by the light harvesting
complex, LH1; cytochrome c is docked against the reaction center.
Right: A dimer of the bc1 complex containing two monomers, each with the three catalytic subunits.
The electrogenic (white arrows) and neutral arms (blue arrows) of the
Mitchellian proton pumps are shown, with the uptake and release of protons
(red arrows) at the catalytic sites.
Physical chemistry of coupling between electron transfer and H+-transport
The physical
chemistry of a model proton pump.
Phosophlipid membranes
A Chime tutorial on a phosopholipid
membrane in the liquid crystalline state using a model from Klaus Schulten's lab. The membrane was generated in a crystalline state by replicating phospholipids in an ordered array to give a bilayer. Waters were added, to give layers several molecules
deep on either side of the membrane. The model was then put through a molecular
dynamics run to simulate heating to give, successively, a gel state (not
shown), and a liquid cystalline state, as shown here. The structure
of water is also of interest in this model, because the H-bonding interactions
to give water chains can be clearly seen (stop the rotation, and zoom in).
These form the basis of the proton
conductivity of water.
Generalized proton circuit
Generalized
diagram of the proton circuits for the major energy conserving electron
transport chains.
The transport of ATP, ADP and phosphate across the mitochondrial membrane
The synthesis or hydrolysis of ATP by intact mitochondria occurs in the
matrix space, because the catalytic sites are on the F1 head
of the ATP synthase lollipop, which projects on the matrix side (N-phase)
of the membrane. Nevertheless, mitochondria are able rapidly to hydrolyse
externally added ATP, and to synthesise ATP outside from added ADP and
phosphate. This is possible because of two transport systems which allow
these metabolites to cross the membrane:
-
The adenine nucleotide transporter.
This enzyme catalyses the exchange of ATP for ADP across the inner
mitochondrial membrane. The charges on the substrates are such that ATP
carries a net excess charge of -1 compared to ADP.
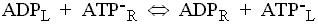
The exchange reaction is therefore electrogenic, and driven by the electrical
component (Dy) of the proton gradient, so that
entry of ADP and exit of ATP are favored.
In, or N-phase 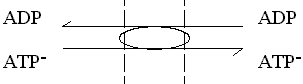 Out, or P-phase
Out, or P-phase
The thermodynamics
of this process, are discussed in more detail here.
-
The phosphate transporter.
The phosphate transporter was the first of the mitochondrial metabolite
transport systems to be demonstrated (Chappell and Crofts, 1965). The enzyme
catalyses the net transfer of H3PO4 across the membrane
in a neutral process. Since the predominant ionic species present at neutral
pH is H2PO4-, the mechanism must involve
either exchange of H2PO4- for OH-,
or cotransport of H2PO4- with H+.
H2PO4-L + OH-R
<==> H2PO4-R + OH-L
or
H2PO4-L + H+L
<==> H2PO4-R + H+R
The exchange allows the chemical component of the proton gradient (-ZDpH)
to drive uptake of phosphate into the mitochondrion.
The net effect of these two transport processes is that the proton
gradient drives the concentration of ADP and phosphate inside the mitochondrion,
and the export of ATP, at the expense of 1H+/ATP synthesized.
More general
aspects of the thermodynamics of transport are covered here.
Ionophores, and transport of ions and metabolites by mitochondria
Early evidence for the chemiosmotic hypothesis came from studies of ion
transport by mitochondria. Three types of transport were studied:
-
Transport of bivalent cations.
When a proton gradient is generated by coupled electron transfer or
ATP-hydrolysis, mitochondrial take up Ca2+ from the external
medium. The Ca2+-uptake is catalysed by a Ca2+ carrier
in the inner membrane which exchanges Ca2+ for H+
(net electrogenicitiy, 1 + charge). When phosphate is also present, a net
uptake of calcium phosphate occurs, which precipitates inside the mitochondria
as hydroxyapatite, visible as dense granules in electron micrographs. If
a weakly acid anion like acetate is present in the suspending medium, calcium
acetate is accumulated, and the mitochondria swell osmotically because
of the increased soluble salt inside.
Transport of Ca2+ plays a physiological role in those
cell processes which are activated or inhibited by changes in Ca2+
activity in the cytoplasm. The mitochondrial transport has a Km
in the physiological range, so uptake buffers the internal [Ca2+].
Mitochondria will transport Mn2+ or Sr2+ in place
of calcium.
-
Metabolite transport.
Mitochondria have diverse metabolic functions, which vary from tissue
to tissue. In liver mitochondria, a wide variety of externally added substrates
can be processed by the mitochondrial metabolic machinery. The enzymes
are predominantly in the matrix, requiring that substrates be transported
across the mitochondrial inner membrane. The transport is catalysed by
a superfamily of proteins, which show strong sequence homology across the
whole spectrum of the eukaryote world, but no obvious prokaryote precursor.
The metabolite transporters generally catalyse neutral exchanges, but some
are electrogenic, or involve H+ cotransport or OH-
exchange, so that the transport equilibrium is determined by the proton
gradient.
Transport
of metabolites will be covered in greater detail later.
-
Ion transport catalysed by ionophores.
Characterization of the mechanisms of ionophores played a crucial role
in our understanding of the proton gradient, and its role in chemiosmotic
coupling. Four ionophoric mechanisms have proved particularly useful:
-
Valinomycin.
Valinomycin catalyses the transprt of K+ (and other monovalent
cations of similar radius like Rb+ or Cs+, but not
Na+) as a charged species across biological membranes (or hydrophobic
phases in general). Transport is by a carrier mechanism. As a consequence,
the electrochemical potential for the transported species is equal across
the membrane, and the equilibrium condition applies.
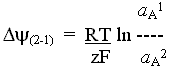
The distribution of ions (usually isotopically labelled Rb+
is use) between inner and outer phases in the presence of valinomycin has
been a favorite method for measuring membrane potential.
The mechanism
of valinomycin will be discussed further in the context of transport.
-
Nigericin.
Nigericin catalyses the neutral exchange of monovalent cations across
biological membranes, with a broad specificity. If a single cation predominates,
the cation is exchanged for H+. As a consequence, when nigericin
is added to mitochondria in the presence of excess K+, the -ZDpH
component of the proton gradient is collapsed. The mechanism
of nigericin is discussed in greater detail here.
-
Gramicidin.
Gramicidin catalyses an electrogenic flux of small monovalent cations,
including protons, across biological membranes through a pore formed by
two molecules end-to-end spanning the membrane. The gramicidin
pore is much studied, and will be discussed in greater
detail later in the context of transport.
As a consequence of the mechanism, gramicidin acts as an uncoupling
agent. In the presence of excess monovalent cation (such as K+),
the transport of K+ is favored over the leak or H+,
and gramicidin will catalyse K+-uptake by mitochondria, similar
to that seen with valinomycin.
-
Protonophores.
Mitchell proposed that uncouplers act by short-circuiting the proton
gradient,- acting as proton conductors. This protonophoric activity has
been much investigated, and the demonstration of a strong correlation between
protonophoric activity in artificial membrane systems, and uncoupling activity,
was a strong piece of evidence favoring the chemiosmotic hypothesis.
Detailed discussion of these useful reagents will be found in Lecture
14.
Respiratory control
In the steady state, the electron transport chain is inhibited by feed-back
from the proton gradient it generates. This results in "respiratory control"
(RC), as seen in the oxygen electrode trace below. The "RC ratio" is the
rate in the presence of ADP and phosphate divided by the rate in the absence
of ADP (or when all ADP has been converted to ATP). The "uncoupled RC ratio"
is the rate in the presence of uncoupler divided by the rate in the absence
of any stimulation by ADP or uncoupler.
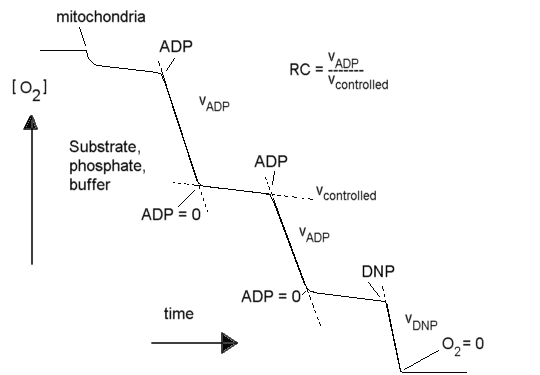
In the absence of reactions to use the proton gradient, the rate of
electron transfer is slower by a factor of ~10 than in the presence of
ADP + phosphate, or an uncoupler (the RC ratio is usually ~10, though the
value depends on substrate). In a system without leaks, the electron transport
chain, the proton gradient, and the ATP synthase reaction would come to
equilibrium, and there would be no flux. The residual electron transfer
is determined by the rate at which protons leak back across the membrane
through non-specific pathways, or the rate at which the various proton
pumps slip the "clutches" which couple their mechanisms to the gradient.
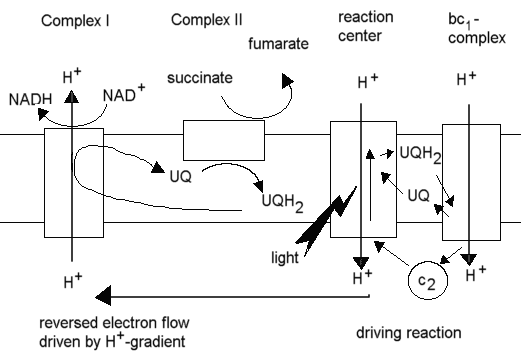
Reversibility of the coupled system,- reversed electron transport
The system normally runs with the redox gradient of the electron transport
chain (for example the DE'oxygen/NADH)
as the driving force, and ATP synthesis as the sink for the energy released.
However, all the reactions of the system are reversible. With the exception
of cytochrome oxidase (where the free energy of the redox reaction cannot
by balanced by that from ATP hydrolysis at physiologically accessible ATP/ADP
ratios), each of the other complexes can be driven in reverse by a driving
force provided either directly by the proton gradient, or indirectly by
ATP hydrolysis through the proton gradient generated.
In the purple photosynthetic bacteria (eg. Rhodobacter sphaeroides),
production of NADH from NAD+ for reduction of metabolites in
the synthetic reactions of the cell occurs through this "reversed electron
flow", with the cyclic photosynthetic chain provided the driving force
through generation of a proton gradient, and succinate acting as the source
of electrons, delivered via the quinone pool to the NADH:UQ oxidoreductase
(Complex I), with the proton gradient driving the latter in reverse, ie.
toward reduction of NAD+.
Equations for transport, chemical and electrochemical potential gradients
Go back to Lecture
9 for a revision of the equations for transport.

©Copyright
1996, Antony Crofts, University of Illinois
at Urbana-Champaign, a-crofts@uiuc.edu


 Out, or P-phase
Out, or P-phase